Genetic variability of memory performance is explained by differences in the brain’s thalamus
The brain’s thalamus has historically been thought of as a relay centre that transmits sensory and motor inputs to the cortex for processing, or that transmits information from one part of the cortex to another. In 2017, three groups made the unexpected discovery that the thalamus also has a key role in short-term memory — specifically, in maintaining the recurrent patterns of cortical activity that underlie memory1–3. However, the genetic basis of this role for the thalamus remained unexplored. Writing in Cell, Hsiao et al.4 reveal that the gene Gpr12 is key to thalamic maintenance of short-term memory. Their findings will have relevance for many fields, from cognitive therapeutics to artificial intelligence.
Perhaps one of the biggest scientific challenges of our time is explaining how intelligent behaviour arises in both natural and artificial systems. Resolving this question will have practical applications. For natural systems, it could allow us to describe and correct behavioural disorders with unprecedented precision. For artificial systems, it would enable safe distribution of agents that will enhance many aspects of our lives, from controlling self-driving cars to fighting misinformation.
Many parallels can be drawn between the two system types, but there are also many differences. For instance, unlike a typical artificial system, the mammalian brain contains organized networks of tight reciprocal connections between two distinct components — the thalamus and the cortex. These two components have different internal structures: neurons in the cortex are highly interconnected, whereas thalamic neurons are not.
In artificial systems, recurrent neural networks can produce short-term memory patterns5. The cortex, at some level of abstraction, can be considered as a collection of recurrent networks that handles different types of short-term memory. So the question arises: why is a thalamus needed in the midst of all of this?
An understanding of the molecular mechanisms that regulate thalamocortical circuits might help us to tackle this question. But identifying genes associated with cognitive processes is hard, because genetic mapping requires many repeated measurements, which can be difficult to obtain from behavioural studies. Hsiao et al. used an innovative approach to overcome this obstacle, making use of a method called quantitative trait locus (QTL) analysis that can link traits (such as eye colour, height or propensity to develop a given disease) to specific locations in the genome, or even to specific genes6.
The team tested the working memory of mice using a simple behavioural task — a maze test, in which the animals explored arms of a T-shaped maze at will. If they chose to explore arms they had not previously visited, they passed the test, whereas if they returned to familiar arms, they failed. The authors found that performance varied between mouse strains, which they reasoned might be partly explained by the ability of individual animals to keep previous actions in mind as short-term memory patterns.
The researchers performed QTL analysis, and found one genetic region that stood out as different between the various strains of mice; they named this region Smart1 (short for spontaneous T-maze alternation QTL 1). In particular, animals that had one particular DNA sequence at Smart1 (dubbed Smart1CAST) were especially good at the exploratory task, and those with another (Smart1B6) were especially poor.
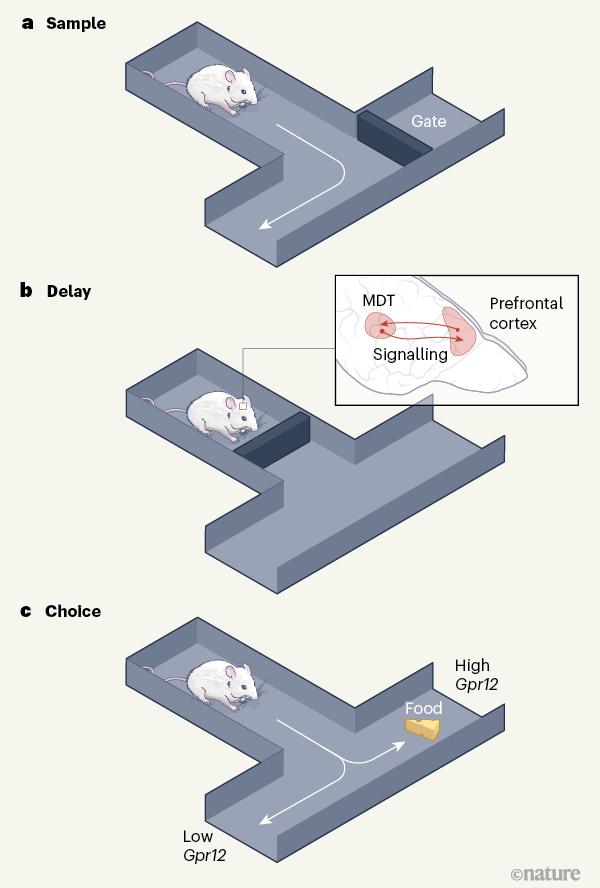
Having identified this region, Hsiao and colleagues confirmed their findings from the high-throughput behavioural test using a similar but more-complex maze assay designed to test spatial working memory. In this assay, which used fewer animals, mice had to remember which arm of a maze they had visited on a first visit, and choose to visit the other arm to get a reward on a second visit (Fig. 1). Again, Smart1CAST and Smart1B6 animals performed better or worse, respectively, than the group as a whole.
Next, Hsiao et al. examined gene-expression patterns across several brain regions in these two mouse strains. The most significant differences between the two were in the mediodorsal thalamus, in expression of a gene called Gpr12 that is located in Smart1. This brain region is strongly connected to the prefrontal cortex, which is involved in higher-level cognitive functions such as working memory. The authors found that reducing expression of Gpr12 led to poorer task performance in Smart1CAST mice, whereas overexpressing the gene improved the performance of Smart1B6 animals.
Gpr12 encodes a protein belonging to a family known as orphan receptors, in which no ligand molecule that binds to and activates each receptor has been identified. Gpr12 probably enhances the activity of mediodorsal thalamus neurons once they are engaged by external inputs (such as those from the prefrontal cortex). Indeed, Hsiao et al. found that patterns of neuronal activity in the mediodorsal thalamus became much more in-sync with those in the prefrontal cortex during those parts of the maze test when animals were presumably remembering where they had been on the previous maze run.
Hsiao and colleagues’ work provides key evidence to reinforce the conclusions of the 2017 papers1–3. Their findings also indicate that coordinated thalamocortical activity patterns depend on the version of Smart1 present: the more Gpr12 is expressed from this region, the more thalamocortical coordination occurs and the better the performance of spatial working memory.
The discovery of this role for Gpr12 could lead to the development of pharmacological agents that boost working-memory performance. However, it would be important to first determine the types of cortical activity pattern that are enhanced by thalamic Gpr12. For example, in tasks in which animals have to withhold actions while remembering a task-relevant piece of information2,7,8, would we see the same type of effect?
It is also intriguing to speculate on what other types of cognitive function could be linked to genetic underpinnings using a QTL approach. The mediodorsal thalamus is known to be involved in switching between tasks9,10; could one find a simple and scalable behavioural test that could be used to assess this process and probe its genetic underpinnings?
Finally, to return to the comparison between natural and artificial systems, is the lack of a thalamus-like architecture in most artificial models of intelligence a missed opportunity? On the one hand, artificial recurrent neural networks require no such structure to maintain memory patterns or switch them across tasks. On the other, perhaps incorporating this biological inspiration into artificial-intelligence systems would enable us to expand their computational capabilities, power efficiency or both. It is exciting to think about the many possibilities ahead as we continue to draw biological inspiration from innovative work such as that of Hsiao and colleagues.
References
- 1.
Bolkan, S. S. et al. Nature Neurosci. 20, 987–996 (2017).
- 2.
Guo, Z. V. et al. Nature 545, 181–186 (2017).
- 3.
Schmitt, L. I. et al. Nature 545, 219–223 (2017).
- 4.
Hsiao, K. et al. Cell 183, 522–536 (2020).
- 5.
Barak, O. Curr. Opin. Neurobiol. 46, 1–6 (2017).
- 6.
Miles, C. M. & Wayne, M. Nature Educ. 1, 208 (2008).
- 7.
Akrami, A., Kopec, C. D., Diamond, M. E. & Brody, C. D. Nature 554, 368–372 (2018).
- 8.
Wimmer, R. et al. Nature 526, 705–709 (2015).
- 9.
Chakraborty, S., Kolling, N., Walton, M. E. & Mitchell, A. S. eLife 5, e13588 (2016).
- 10.
Rikhye, R. V., Gilra, A. & Halassa, M. M. Nature Neurosci. 21, 1753–1763 (2018).